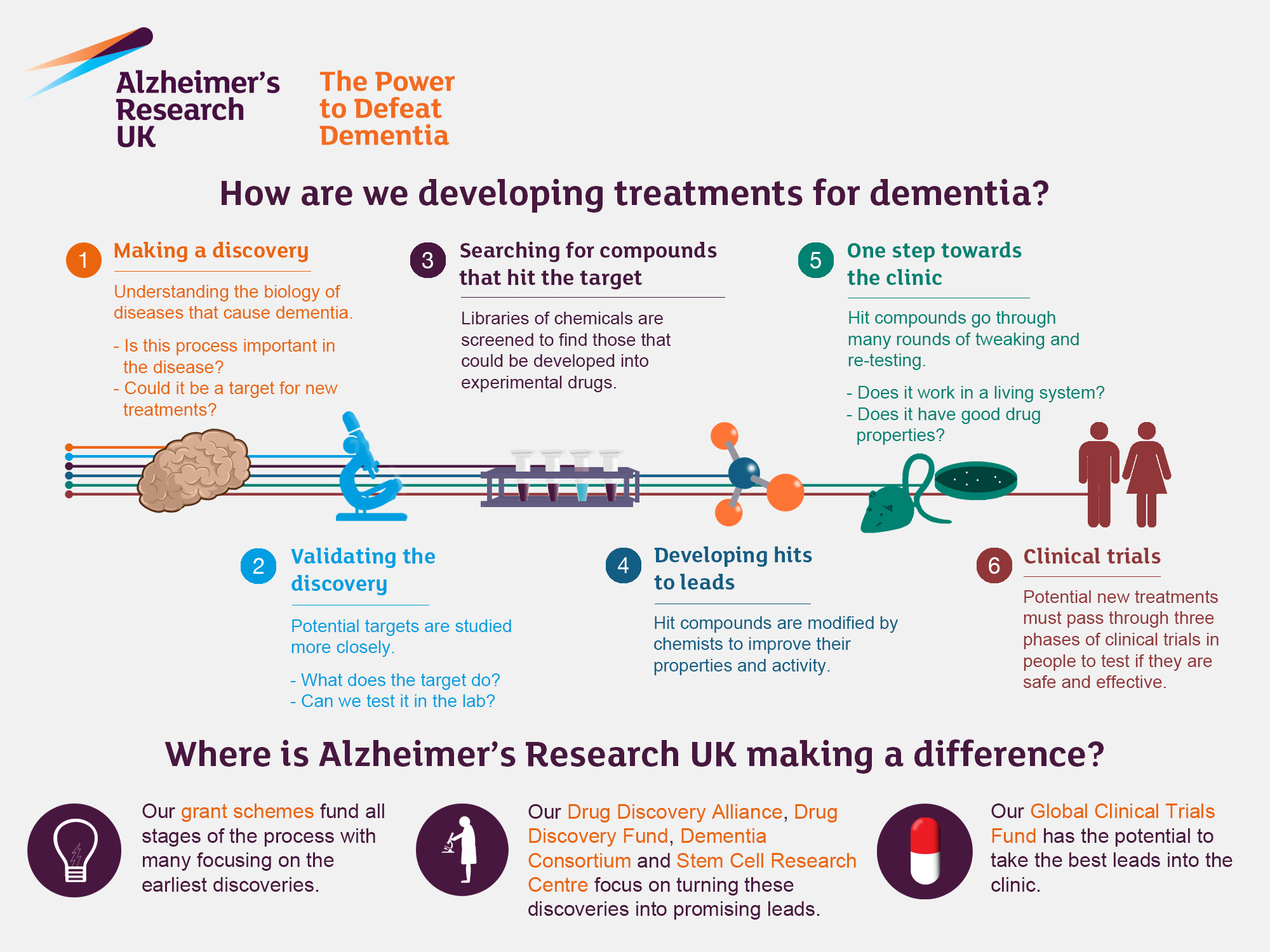
Alzheimer’s research has seen a transformative shift, thanks to the pioneering work of scientists like Beth Stevens, who delve into the complexities of neurodegenerative diseases. By focusing on microglial cells, which act as the brain’s immune system, researchers are uncovering crucial insights into how these immune cells can inadvertently contribute to Alzheimer’s disease. The abnormal pruning of synapses — a process essential for a healthy brain — can lead to cognitive decline when dysregulated. This groundbreaking research not only informs new Alzheimer’s treatment strategies but also paves the way for developing biomarkers essential for early detection. With roughly 7 million Americans affected by this debilitating condition, understanding the dynamics of microglial function may be key to unlocking effective therapies and ultimately improving patient care.
The exploration of Alzheimer’s pathology encompasses a wide range of scientific inquiries into age-related cognitive disorders. This field is critically involved in examining how various immune mechanisms, particularly those governed by the brain’s immune cells known as microglia, influence neurodegenerative processes. Researchers like Stevens are uncovering how these cells participate in both the healthy maintenance and the damaging progression of diseases such as Alzheimer’s and Huntington’s. By targeting these immune pathways, innovative strategies for prevention and intervention are beginning to emerge, offering hope to millions affected. Additionally, exploring how neuroinflammation correlates with cognitive decline is vital for developing comprehensive approaches to manage and treat these complex conditions.
Understanding Microglial Cells and Their Role in Neurodegenerative Diseases
Microglial cells, often referred to as the brain’s immune system, play a crucial role in maintaining neurological health. These specialized cells constantly survey the brain environment, identifying and eliminating damaged neurons and debris resulting from cellular injuries. The importance of microglia cannot be overstated, particularly in the context of neurodegenerative diseases like Alzheimer’s. Recent research by Beth Stevens has illuminated how these cells contribute to synaptic pruning during both brain development and diseases. An imbalance in this process can result in excessive synaptic loss, a hallmark of Alzheimer’s, which underscores the complexity of microglial functions in neurodegenerative conditions.
In the quest to understand Alzheimer’s disease, the investigation of microglial activity has revealed new insights into how the brain regulates neuronal connections. Abnormal microglial responses may lead to pathological synaptic pruning, exacerbating cognitive decline in Alzheimer’s patients. Studies have shown that these immune cells, when activated incorrectly, can initiate a cascade of inflammatory responses detrimental to neuron survival. As such, targeting microglial dysfunction represents a promising avenue for developing therapies aimed at mitigating the effects of neurodegenerative diseases, emphasizing the need for further research in this area.
The Impact of Beth Stevens’ Research on Alzheimer’s Treatment
Beth Stevens’ pioneering work on microglial cells has greatly expanded our understanding of Alzheimer’s treatment strategies. By unraveling the complex interactions between microglia and neuronal health, her research has laid the groundwork for the development of novel biomarkers. These biomarkers can potentially provide early detection of Alzheimer’s, allowing for timely therapeutic interventions. As the population of individuals affected by Alzheimer’s disease continues to rise, the importance of such research becomes even more critical, presenting hope for better management and treatment options for millions of families.
Furthermore, Stevens emphasizes the significance of federal support for scientific research, highlighting that much of her groundbreaking work has been funded by the National Institutes of Health (NIH). This funding is instrumental in allowing researchers to pursue curiosity-driven science, which often leads to unforeseen discoveries that advance our understanding of diseases like Alzheimer’s. As we aim to enhance the current Alzheimer’s treatment landscape, the integration of findings from microglial cell studies may open new pathways for innovative therapeutic approaches that could significantly improve the quality of life for patients.
The Future of Alzheimer’s Research: Bridging Basic Science and Clinical Applications
The future of Alzheimer’s research hinges on bridging the gap between basic science and clinical applications. As demonstrated by the work of scientists like Beth Stevens, foundational research is essential for uncovering the cellular mechanisms underlying neurodegenerative diseases. This exploration not only informs us about the biological basis of conditions like Alzheimer’s but also guides the development of targeted therapies that can alter disease trajectories. By focusing on the role of microglial cells, researchers can develop strategies that address not only symptoms but also the pathological processes central to disease progression.
Moreover, the dialogue between researchers and clinicians is vital for translating discoveries into effective Alzheimer’s treatments. Engaging clinical perspectives can ensure that research aligns with patient needs, ultimately leading to more practical applications of basic science findings. Initiatives aimed at accelerating the translation of discoveries from the lab to the clinic are crucial. As the understanding of the brain’s immune system evolves, it holds the potential to revolutionize the treatment of Alzheimer’s, marking a significant step forward in combating this debilitating disease.
Synaptic Pruning: A Double-Edged Sword in Alzheimer’s Disease
Synaptic pruning is a natural process essential for brain development and function, but when it becomes dysregulated, it can lead to catastrophic outcomes such as those observed in Alzheimer’s disease. As highlighted in Beth Stevens’ research, microglial cells are responsible for this critical process, selectively eliminating synapses that are no longer beneficial. However, in neurodegenerative diseases, excessive pruning can occur, which is detrimental to cognitive function. This paradoxical role of synaptic pruning underscores the complexity of brain immune responses and their implications for Alzheimer’s disease.
Understanding the mechanisms underlying synaptic pruning is pivotal for developing targeted interventions to correct maladaptive pruning in Alzheimer’s patients. By leveraging insights from microglial research, scientists aim to create therapeutic strategies that can restore normal synaptic architecture, potentially reversing some of the cognitive declines associated with the disease. This area of research not only provides a deeper comprehension of Alzheimer’s but also paves the way for innovative treatments that can enhance neuronal resilience and preserve cognitive functions.
Exploring New Biomarkers for Early Detection of Alzheimer’s Disease
The identification of novel biomarkers is a critical focus in Alzheimer’s research, as early detection significantly enhances treatment efficacy. Beth Stevens’ exploration of the relationship between microglial activity and synaptic health contributes to this emerging field of biomarker development. By understanding how microglial cells interact with neurons during the onset of Alzheimer’s pathology, researchers can identify specific molecular signatures indicative of early disease processes. Such advancements may facilitate timely interventions that can slow down or potentially halt disease progression.
Moreover, these biomarkers could provide invaluable tools for clinical trials, helping to stratify patient populations based on their disease stage or underlying molecular characteristics. With the continuing rise of Alzheimer’s prevalence worldwide, the capacity to detect the disease at its nascent stage not only aids research efforts but also holds promise for enhancing patient outcomes. Therefore, investment in biomarker research is crucial, ensuring that discoveries in basic neuroscience quickly translate into benefits for Alzheimer’s patients.
Innovative Approaches to Targeting Inflammation in Alzheimer’s Disease
Inflammation has long been recognized as a contributing factor in the pathology of Alzheimer’s disease, with microglial cells at the center of this ongoing research. Beth Stevens’ work provides significant insight into how neuroinflammation can influence neuronal health and synaptic integrity. By elucidating the role of microglia in the inflammatory processes associated with Alzheimer’s, her research opens up the possibility for developing innovative anti-inflammatory strategies aimed at mitigating disease progression. This approach is particularly promising as it addresses one of the crucial mechanisms contributing to neurodegeneration.
Targeting inflammation through modulating microglial activity presents an exciting frontier in Alzheimer’s treatment. Ongoing studies are exploring various compounds aimed at recalibrating microglial function, with hopes of restoring their protective roles while curtailing their contributions to neurodegeneration. The challenge lies in balancing the microglial responses to avoid unwanted exacerbation of inflammation, but advancements in our understanding of these cells could lead to breakthrough therapies that enhance brain resilience against Alzheimer-related decline.
Advances in Neurotherapy: Learning from Microglial Research
Advancements in neurotherapy derive immense benefits from the increasing knowledge surrounding microglial cells and their influence on brain health. Through ongoing research, including the significant contributions of Beth Stevens, we are gaining a clearer picture of how nurturing microglial functions can lead to improved outcomes for individuals suffering from Alzheimer’s and other neurodegenerative diseases. Targeting microglial pathways has the potential to transform how we approach treatment, offering new avenues for therapies that focus on maintaining synaptic integrity and neuronal health.
This neurotherapeutic potential is bolstered by innovations in drug development aimed at enhancing the beneficial aspects of microglial activity while attenuating the harmful responses associated with neuroinflammation. As researchers pursue this dual approach, the possibility of developing not only disease-modifying therapies but also symptomatic relief options becomes increasingly feasible. Such advancements signal a progressive shift in the landscape of Alzheimer’s treatment, where microglial research plays a central role in driving therapeutic innovations.
The Importance of Federal Funding in Alzheimer’s Research
Federal funding is a cornerstone of Alzheimer’s research, enabling scientists like Beth Stevens to explore complex questions about brain health and disease. Support from agencies such as the NIH provides essential resources that allow researchers to pursue their inquiries without the constraints often posed by limited budgets. This financial backing makes a significant difference in advancing scientific understanding, particularly in emerging fields like microglial research that promise novel insights into neurodegenerative disorders.
Moreover, robust federal investment in Alzheimer’s research signals a commitment to tackling this pressing public health crisis. By facilitating innovative studies and supporting researchers, funding opportunities not only enhance scientific discovery but also pave the way for future treatments. The continued emphasis on curiosity-driven research fosters an environment where groundbreaking advancements can emerge, ultimately benefitting the millions impacted by neurodegenerative diseases like Alzheimer’s.
The Role of Curiosity-Driven Research in Alzheimer’s Discoveries
Curiosity-driven research forms the backbone of significant scientific advancements, as illustrated by the successes in understanding Alzheimer’s disease through the lens of microglial activity. Beth Stevens’ journey reflects how following scientific curiosity can lead to transformative discoveries that reshape our understanding of neurological conditions. By delving into the unknown—examining the functions of microglia—researchers uncover insights that not only enhance our understanding of Alzheimer’s but open new avenues for treatment and prevention strategies.
The beauty of curiosity-driven research lies in its potential to yield unexpected results that can influence broad swaths of medicine. As Stevens emphasizes, seemingly unrelated studies can eventually lead to groundbreaking treatments, demonstrating the intricate connections within biological systems. By fostering a research culture that prioritizes curiosity, we can continue to unravel the complexities of Alzheimer’s disease and, eventually, herald significant breakthroughs in how we approach treatment and care for affected individuals.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are essential components of the brain’s immune system and play a significant role in Alzheimer’s research. They help remove dead or damaged cells and prune synapses, which are crucial for neuron communication. However, abnormal microglial activity can contribute to neurodegenerative diseases like Alzheimer’s, making them a key focus for developing new treatments and biomarkers.
How is Beth Stevens contributing to advancements in Alzheimer’s treatment?
Beth Stevens, a prominent neuroscientist, has made significant contributions to Alzheimer’s treatment through her research on microglial cells. Her findings reveal how these cells can both protect and harm brain function, leading to insights that shape potential therapies for Alzheimer’s and other neurodegenerative diseases.
What is the connection between neurodegenerative diseases and the brain immune system?
Neurodegenerative diseases, including Alzheimer’s, are often linked to dysfunction in the brain’s immune system, particularly through the action of microglial cells. These cells respond to injury and inflammation but can also become detrimental when they mistakenly prune healthy neuronal connections, exacerbating disease progression.
Why are microglial cells considered a focus in Alzheimer’s research?
Microglial cells are a focal point in Alzheimer’s research because they act as the brain’s immune responders. Recent studies have shown that their activity in removing damaged cells and synapses can turn pathological, contributing to the disease’s progression. Understanding these mechanisms may lead to new therapeutic approaches.
Can understanding microglial function lead to breakthroughs in Alzheimer’s treatment?
Yes, understanding microglial function is crucial for breakthroughs in Alzheimer’s treatment. Research by scientists like Beth Stevens has demonstrated how these immune cells interact with neurons and influence neurodegenerative processes. This knowledge is paving the way for developing innovative therapies to slow or halt Alzheimer’s disease.
What are the implications of abnormal microglial activity in Alzheimer’s?
Abnormal microglial activity can have serious implications for Alzheimer’s disease, including the excessive removal of synapses, which impairs neuronal communication. This dysregulation contributes to cognitive decline and pathophysiological changes associated with Alzheimer’s, making microglial cells an important target for therapeutic intervention.
How are NIH grants supporting Alzheimer’s research focused on microglial cells?
NIH grants provide essential funding for Alzheimer’s research focused on microglial cells, supporting projects that explore their roles in brain health and disease. This funding helps researchers like Beth Stevens investigate how microglial dysfunction contributes to neurodegenerative diseases, fostering new discoveries that may lead to effective treatments.
What discoveries have emerged from Beth Stevens’ research on microglial cells?
Beth Stevens’ research has led to groundbreaking discoveries about the role of microglial cells in synaptic pruning and brain health. Her work suggests that understanding these cells’ function can illuminate pathways involved in Alzheimer’s disease, laying the groundwork for new biomarkers and potential therapies.
How does ongoing Alzheimer’s research impact the 7 million Americans living with the disease?
Ongoing Alzheimer’s research, particularly studies focused on microglial cells and neurodegenerative mechanisms, holds promise for the 7 million Americans living with the disease. By uncovering new insights and developing targeted therapies, researchers aim to improve diagnosis, treatment, and ultimately, the quality of life for individuals affected by Alzheimer’s.
What is the importance of foundational research in Alzheimer’s studies?
Foundational research is critical in Alzheimer’s studies as it lays the groundwork for understanding complex biological processes, such as those involving microglial cells. This type of research may not provide immediate results but is essential for driving innovation and breakthroughs in treatments for neurodegenerative diseases like Alzheimer’s.
| Key Point | Detail |
|---|---|
| Research by Beth Stevens | Under NIH support, focusing on Alzheimer’s and related disorders. |
| Role of Microglial Cells | Act as the brain’s immune system, removing harmful cells and pruning synapses. |
| Impact on Alzheimer’s Disease | Abnormal microglial pruning linked to Alzheimer’s and other neurodegenerative disorders. |
| New Biomarkers and Medications | Research leads to new methods for detecting and treating neurodegenerative diseases. |
| Funding and Support | Stevens’ research benefitted greatly from NIH funding throughout her career. |
| Long-term Research Goals | Studies in foundational science are crucial for breakthroughs in understanding diseases. |
Summary
Alzheimer’s research has gained momentum through the groundbreaking discoveries made by scientists like Beth Stevens, who have unveiled the critical functions of microglial cells in the brain’s immune response. By understanding how these cells operate and the implications of their abnormal processes, researchers are paving the way for innovative treatments and interventions that hold the potential to transform the lives of millions affected by Alzheimer’s disease.