Patient safety in medical research is a fundamental concern that influences the integrity and efficacy of clinical trials and studies across the globe. As medical research funding, especially from organizations like the NIH, undergoes cuts, the mechanisms that ensure participant protection may be severely compromised. With IRB oversight crucial for evaluating and monitoring research activities, the cessation of these funds poses a direct threat to the ethical standards that govern clinical trial ethics. The nuanced interplay between planning, risk assessment, and ethical compliance is critical in safeguarding the rights of individuals who volunteer for studies. If we do not prioritize patient safety, the repercussions could extend beyond the research environment, shaking public trust in the very systems designed to protect them.
In the realm of health sciences, safeguarding individuals engaged in research is paramount, encompassing their rights and well-being throughout the study process. The significance of participant protection is reflected in the rigorous frameworks established by oversight bodies, such as Institutional Review Boards (IRBs), which are responsible for ensuring ethical conduct in research involving human subjects. Funding for these essential oversight mechanisms not only supports clinical trial ethics but also facilitates open collaboration among institutions involved in medical investigation. As we delve into the complexities surrounding the financial and procedural elements of medical research, understanding how these factors interlace with participant welfare will help underscore the vital importance of maintaining rigorous safety standards.
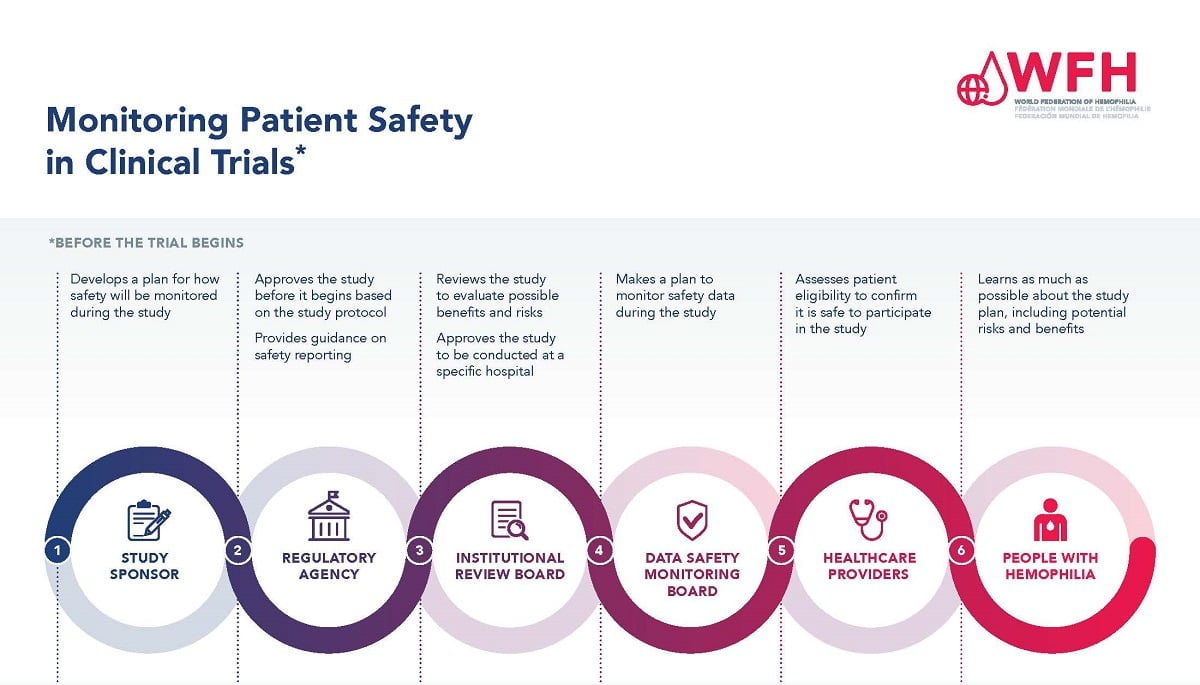
The Role of IRB Oversight in Medical Research
Institutional Review Boards (IRBs) serve as a vital component of the medical research landscape, playing a critical role in the oversight and ethical review of studies involving human participants. By ensuring compliance with federal regulations and institutional policies, IRBs prioritize participant protection and safety. These boards meticulously assess research protocols, examining aspects such as informed consent, risk assessment, and data monitoring, which underpins the integrity of clinical trials. As per NIH guidelines, particularly for federally funded research, oversight by an IRB is not just a bureaucratic necessity but a fundamental ethical obligation that safeguards the rights of research participants.
Moreover, IRBs contribute significantly to the quality and credibility of research outcomes. By acting as a checkpoint for ethical considerations, they help maintain public trust in medical research. The evolution of IRBs reflects a historical response to past abuses in clinical trials, making them indispensable in today’s complex research environment. As research increasingly involves multisite collaborations, adherence to IRB guidelines across various institutions, especially through systems like SMART IRB, enhances patient safety and ensures standardized ethical oversight.
Impact of Funding Cuts on Patient Safety
The recent freeze on federal research funding, particularly the more than $2 billion cut to Harvard, has raised serious concerns about patient safety in ongoing clinical studies. Research that primarily seeks to enhance patient care and public health is jeopardized when funding is slashed, leading to delays and interruptions in critical projects. For instance, the halt of supporting resources prevents IRBs from effectively managing the oversight of concurrent studies, creating a ripple effect that adversely affects participant engagement and safety. As participant protection measures become less robust, the ethical principles underlying these studies are put at risk, potentially harming those volunteers invested in advancing medical knowledge.
Without adequate funding, the ability of IRBs to oversee research diminishes, affecting the entire research ecosystem. Delays in clinical trials due to funding shortages can undermine the confidence of the public in research initiatives, further complicating recruitment efforts for future studies. As researchers struggle to navigate funding challenges, the ethical conduct of research may falter, leading to compromised safety protocols. Ultimately, ensuring that necessary funding channels are restored is crucial for preserving the integrity of medical research and, most importantly, the safety of patients.
Ensuring Participant Protection in Clinical Trials
Participant protection remains a cornerstone of ethical medical research, emphasizing the necessity for informed consent and ongoing support for those involved in clinical trials. It is critical that potential research participants are fully aware of their rights, the nature of the studies, and any risks involved before they consent to participate. Moreover, the role of IRBs is pivotal in evaluating the adequacy of these consent processes. Providing clear, comprehensive information fosters trust and encourages individuals to partake in essential research that can lead to medical advancements.
Additionally, IRBs not only oversee the research protocol but also monitor the welfare of participants throughout the study. This continuous oversight helps in identifying and addressing any adverse events that may arise, ensuring that participant safety is prioritized at all times. By engaging with researchers to uphold ethical standards and advocating for participant rights, IRBs contribute significantly to the integrity and ethical foundation of clinical research.
The Consequences of Funding Cuts on Patient Health
The consequences of funding cuts in medical research extend beyond financial implications; they threaten the very health of participants involved in various studies. When vital research projects are stalled or cancelled, it directly impacts not only the participants but also the broader community that stands to benefit from new treatments and medical breakthroughs. The halt in critical studies, especially for chronic diseases or pressing health challenges, can lead to delayed access to innovative therapies and potentially critical medication.
Moreover, financial disruptions can create a chilling effect on clinical trial participation, as potential volunteers may perceive a less reliable environment for their health and safety. This decline can significantly hinder advancements in medical research, as reduced participation limits the sample sizes necessary for generating credible and generalizable findings. Ultimately, the continuity of funding is essential not just for supporting individual research projects but for fostering public health and enhancing patient care across diverse medical fields.
The Importance of NIH Research Grants
NIH research grants serve as a crucial source of funding that supports a wide array of medical research initiatives aimed at improving patient outcomes. These grants are designed to finance studies that often require extensive resources, from basic scientific inquiries to complex clinical trials. Through proper allocation of NIH funds, researchers are better equipped to uphold participant safety standards and conduct thorough ethical reviews, ultimately leading to advancements in medical science that benefit society.
Additionally, NIH grants play a critical role in empowering institutions like Harvard to maintain their commitment to rigorous oversight through programs such as SMART IRB. These grants not only provide the financial backing necessary for research activities but also promote collaborative efforts across institutions, ensuring that participant protection remains at the forefront of clinical trials. Maintaining and enhancing NIH funding is essential for sustaining an environment where ethical medical research can thrive, thereby fostering innovation that translates into better health outcomes.
Strengthening Participant Rights in Research
As medical research evolves, strengthening participant rights becomes increasingly paramount to uphold ethical standards and ensure informed consent. Participants are not merely subjects; they are partners in the research process, and their rights must be prioritized at every stage. Ensuring that participants are fully informed about the risks and benefits of their involvement cultivates an environment of trust and respect. It also reinforces their autonomy, allowing them to make informed decisions regarding their participation.
Moreover, modern research requires IRBs to focus on empowering participants through education and transparency. By actively engaging with potential volunteers and providing them with comprehensive information regarding study protocols, researchers and IRBs reaffirm their commitment to participant protection. Enhancing training programs for researchers on informed consent practices can further support the ethical conduct of studies and promote a culture of accountability and integrity within the research community.
Historical Context of IRBs and Patient Safety
Understanding the historical context in which IRBs were established highlights the fundamental importance of patient safety and ethical oversight in medical research. The legacy of past abuses, such as the Tuskegee Syphilis Study and the unethical experimentation during WWII, significantly shaped the development of institutional safeguards that protect research participants today. These historical failures serve as stark reminders of the need for rigorous oversight to ensure that every participant is treated with dignity and respect, reinforcing a commitment to ethical practices in all research initiatives.
The creation of IRBs was a direct response aimed at preventing such violations from occurring in modern clinical trials. These oversight bodies are specifically tasked with scrutinizing research proposals to assess their ethical implications and ensure that appropriate measures are in place to protect participant welfare. As we navigate the complexities of contemporary medical research, the lessons learned from history serve as a guiding framework that underscores the necessity of robust ethical reviews to uphold the safety and rights of all individuals involved.
The Interplay Between Research Funding and Participant Safety
The interplay between research funding and participant safety is critical to the success of medical studies. Adequate funding not only enables the execution of rigorous research protocols but also ensures that robust systems are in place to protect participants throughout the study. When research funding is compromised, the resources available for comprehensive ethical oversight diminish, potentially jeopardizing the safety and rights of those who volunteer for clinical trials.
Furthermore, the funding landscape influences the ability of institutions to implement effective IRB oversight and training programs that enhance participant protection. As funding cuts limit the capacity to hire and retain qualified personnel, the effectiveness of research organizations in maintaining high ethical standards may wane. Therefore, advocating for sustained research funding is vital to preserving a critical infrastructure that supports patient safety and ethical accountability within medical research.
Future Directions in Medical Research Ethics
As we look to the future, establishing a robust ethical framework for medical research will be essential for enhancing patient safety and public trust. Emphasizing the importance of participant involvement and advocacy within the research process can significantly strengthen ethical practices. By actively engaging participants in conversations about their needs and concerns, researchers can develop protocols that prioritize safety and ethical integrity. Furthermore, ongoing education for researchers regarding ethical standards will be crucial to adapting to evolving challenges in medical research.
The future also holds potential for increased transparency and collaboration among research institutions, particularly in the wake of funding changes. Initiatives aimed at standardizing IRB processes across institutions and sharing best practices can foster an environment of accountability and shared responsibility for patient safety. By leveraging technology and fostering partnerships within the research community, we can enhance the ethical oversight of studies and ensure that the safety and well-being of participants remain central to all medical research endeavors.
Frequently Asked Questions
How does IRB oversight impact patient safety in medical research?
IRB oversight is crucial for ensuring patient safety in medical research by rigorously reviewing and approving research proposals. Institutional Review Boards (IRBs) assess participant protection, informed consent processes, risk management, and ethical compliance, which collectively safeguard the rights and welfare of research participants throughout the study.
What are the implications of funding cuts on participant protection in medical research?
Cuts to medical research funding can severely hinder participant protection efforts. Reduced funding limits the capacity of IRBs to conduct thorough reviews and oversight, potentially exposing patients to unaddressed risks. Furthermore, halted studies can erode public trust in research, ultimately impacting participant recruitment and the overall integrity of medical research.
How do NIH research grants contribute to the ethics of clinical trials?
NIH research grants play a vital role in promoting ethical clinical trials by requiring stringent IRB oversight for studies involving human participants. These grants facilitate compliance with regulations designed to protect participants, ensuring that risks are minimized and that ethical standards are maintained throughout the research process.
What systems are in place to ensure patient safety during multi-site medical research?
In multi-site medical research, systems like the SMART IRB facilitate patient safety by providing a streamlined review process through a single IRB. This collaboration ensures consistent oversight across all participating sites, optimizing participant protection, maintaining ethical standards, and preventing duplication of efforts that could compromise research integrity.
Why is participant protection a critical aspect of clinical trial ethics?
Participant protection is fundamental to clinical trial ethics because it ensures that individuals involved in research are treated with respect and their rights are upheld. This includes obtaining informed consent, continuously monitoring trial impacts on participants, and addressing any potential adverse effects, which are essential for maintaining public trust in the medical research community.
What historical events led to the establishment of IRBs for patient safety in medical research?
IRBs were established in response to historical abuses in medical research, such as the Tuskegee Syphilis Study and unethical experiments during WWII. These events highlighted the urgent need for ethical oversight to protect human subjects. Consequently, regulations were formed to ensure informed consent and participant safety, foundational elements of modern clinical research ethics.
How does IRB oversight evolve in response to new challenges in medical research?
IRB oversight evolves by adapting to emerging ethical challenges and technological advancements in medical research. Continuous training for IRB members, updating of procedures, and integration of public feedback are essential for maintaining high standards of participant protection and ensuring compliance with ever-changing laws and ethical norms.
What role do individuals and communities play in ensuring patient safety in medical research?
Individuals and communities are pivotal in ensuring patient safety by participating in clinical trials and providing feedback on research practices. Their involvement helps to uphold ethical standards and reinforce trust in medical research, ultimately guiding IRBs and researchers to prioritize the welfare of research participants.
| Key Point | Details |
|---|---|
| Funding Cuts Impact | The Trump administration’s freeze of $2 billion in federal research grants disrupts patient safety efforts. |
| Role of IRB | IRBs ensure compliance and protect patient rights in research by reviewing proposals and overseeing studies. |
| SMART IRB | A national system to facilitate oversight across multiple research sites has faced a halt in operations due to funding issues. |
| Historical Context | Past unethical research practices led to the establishment of IRBs and the commitment to patient safety in medical research. |
| Risks of Halting Research | Funding cuts lead to delayed studies, raising public skepticism and potential harm to participants. |
Summary
Patient safety in medical research is significantly threatened by funding cuts that disrupt crucial oversight systems such as SMART IRB. With the halt of over $2 billion in federal research grants, efforts to protect the rights and welfare of participants are compromised, leading to potential delays in research and increased risks for patients. This situation undermines public trust and diminishes the effectiveness of institutional review boards that play a vital role in safeguarding patient safety. Hence, it is imperative to address these funding challenges to ensure the continued protection of those involved in medical research.