Medical research oversight plays a critical role in ensuring that clinical studies prioritize patient safety and uphold medical ethics. This system is essential for protecting the rights and welfare of individuals who volunteer as participants in research, particularly when considering the complexities of the IRB process. In light of recent funding cuts, such as the more than $2 billion freeze in federal research grants to Harvard, the integrity of clinical research is at risk. Without adequate oversight, the potential for harm to patients increases, undermining trust in the research community and its mission to advance healthcare. As the landscape of research funding evolves, understanding the implications of these changes on patient safety in research becomes paramount for all stakeholders involved in the clinical research enterprise.
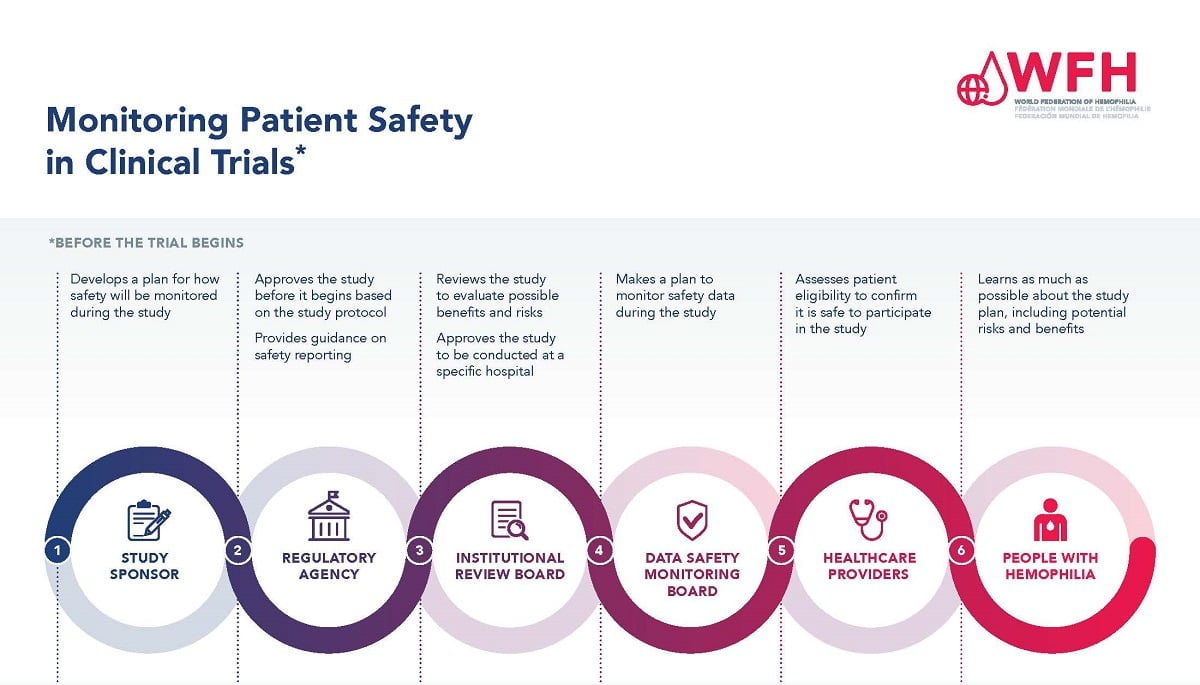
Oversight in medical research encompasses a variety of processes and institutions crucial for maintaining ethical standards and safeguarding participants. Often referred to as research governance, this framework ensures that studies comply with a range of regulations and ethical norms designed to protect study subjects. With increasing collaboration across multiple sites, the importance of streamlined review mechanisms, such as that provided by institutional review boards (IRBs), cannot be overstated. These governing bodies are pivotal in reviewing projects to maintain clinical research integrity and ensure that the rights of individuals participating in studies are fiercely upheld. As medical research continues to evolve, the intersection of funding, patient safety, and ethical oversight remains a focal point of discourse within the scientific community.
Impact of Funding Cuts on Medical Research Oversight
The recent halt in federal funding has sent shockwaves through the landscape of medical research oversight, particularly affecting the institutional review boards (IRBs) that ensure patient safety. With Harvard’s federal research grants frozen, the SMART IRB system, which is vital for coordinating oversight across multiple sites, is now at risk. This disruption not only threatens the ongoing studies but also undermines the overall integrity of the clinical research process. As the IRB process faces additional strain from funding cuts, fewer resources will be available to monitor compliance with medical ethics, putting patient welfare in jeopardy.
Moreover, the inability of research institutions to collaborate effectively hampers the collective effort needed to address pressing health concerns. When research sites are unable to add new clinical trials or share resources due to funding restrictions, the potential for breakthroughs in treatment diminishes. The continuity of oversight is paramount in maintaining public trust in clinical trials. As studies are delayed or canceled, concern among the public grows, which further impacts patient participation in future research. Reinforcing these oversight mechanisms is crucial to ensuring that patient safety and the integrity of research practices remain at the forefront.
The Role of Institutional Review Boards in Ensuring Patient Safety
Institutional review boards (IRBs) play an essential role in safeguarding the rights and well-being of participants in clinical studies. By thoroughly reviewing research proposals, IRBs assess potential risks and ensure that proper informed consent procedures are in place. This review process is integral to adhering to medical ethics and promoting transparency within clinical research. When the proper oversight is in place, it not only protects participants but also enhances the integrity of the research findings, fostering more significant advancements in medical science.
The dynamic of IRBs involves constant interaction with researchers, ensuring that there is ongoing education about patient rights and safety protocols. They serve as a bridge between the research community and the general public, especially when it comes to addressing any adverse events or ethical dilemmas that might arise during studies. Given the historical context of medical ethics, where breaches of trust have led to significant consequences, the protective measures employed by IRBs are vital in rebuilding and maintaining confidence in medical research across communities.
The Consequences of Disrupted Funding on Patient Engagement
When funding for medical research is curtailed, the ripple effects can drastically undermine patient engagement in clinical trials. With fewer studies being conducted, potential participants may feel less inclined to volunteer, believing that the research efforts are not meeting the necessary standards for safety and ethics. This hesitation can be compounded by a lack of information regarding how funding cuts directly affect the integrity of research processes and their ability to protect patient interests. The subsequent decline in participation can then distort results, leading to inadequate data and incomplete knowledge about treatment efficacy.
Additionally, as funding becomes scarcer, it may lead to a tendency for researchers to cut corners, potentially compromising patient safety and ethical standards. A system that relies heavily on public trust can only thrive when participants feel assured of their rights and welfare in research settings. When funding disruptions hinder the activities of IRBs and training programs for researchers, the foundational structures that support patient safety in research become weakened. As a consequence, this can lead to a retraction of critical support from communities that are crucial for successful trial completion.
Understanding NIH Funding and Its Impact on Clinical Research
The National Institutes of Health (NIH) is a cornerstone of funding for medical research in the United States, supporting countless studies aimed at understanding and treating various conditions. When resentments rise against NIH funding policies, such as those seen during the recent stop-work order on research grants, the implications are dire for clinical research integrity. The NIH mandates that all studies including human participants undergo stringent IRB reviews, which are now at risk of being delayed or eliminated due to funding constraints.
Moreover, NIH funding significantly contributes to the overall safeguards in place for patient safety. This funding allows for the establishment of protocols that protect individuals participating in studies, ensuring that risks are minimized and ethical standards are upheld. The suspension of such funds not only disrupts existing studies but also inhibits the broader research community from advancing their work on pressing medical issues. As funding continues to be a pivotal factor, understanding its role in ensuring compliance with medical ethics becomes increasingly essential.
Historical Significance of Medical Ethics in Research Oversight
The evolution of medical ethics is deeply rooted in historical events that have shaped contemporary research oversight. From the atrocities committed during World War II to the unethical practices seen in the Tuskegee syphilis study, these examples underscore the critical need for stringent oversight in medical research. IRBs were established as a response to these historical failures, instituting a framework that safeguards against exploitation and abuse, ensuring that patient rights are respected. The principles established in response to these events continue to guide modern research ethics, enabling a more humane and respectful approach to medical inquiries.
As we reflect on these past transgressions, it becomes clear that the regulations and oversight mechanisms in place today are not merely bureaucratic obstacles but vital protections for study participants. The establishment of ethics committees and IRBs has been a monumental achievement in ensuring that research is not conducted at the expense of individuals’ rights and dignity. As funding cuts threaten to dismantle the infrastructure supporting these essential protections, it is crucial to advocate for the preservation and enhancement of these ethical safeguards.
Community Trust and the Impact of Research Funding Disruptions
Trust is a critical component of successful clinical research, and disruptions in funding can severely erode this trust. When research studies are halted or significantly delayed, communities may begin to view clinical trials with skepticism. This mistrust can stem from concerns over patient safety, exacerbated by the uncertainty caused by funding gaps. The historical context of exploitation in medical research leaves a legacy of suspicion that necessitates continuous efforts to foster transparency and accountability among researchers and institutions.
Furthermore, maintaining the bond between researchers and the communities they serve is vital for future research initiatives. The cessation of collaborative research efforts due to funding disruptions not only impedes progress but also alienates potential participants. When communities perceive that their involvement in research may be jeopardized, their willingness to engage diminishes. To rebuild trust, it is imperative to demonstrate how patient safety is prioritized and to emphasize the ethical frameworks that guide research conduct, particularly in times of financial uncertainty.
Strengthening Mechanisms for Patient Protection in Research
To ensure patient safety in the face of funding challenges, it is essential to strengthen the mechanisms by which research oversight operates. This includes bolstering IRBs and other ethical committees that serve as the backbone of patient protection. By increasing training and resources for these entities, the capacity to monitor compliance with ethical standards can be enhanced, ensuring that the rights and welfare of participants remain the top priority. Allocating funding specifically for the education and operational support of these oversight bodies can help mitigate the risks associated with funding cuts.
Additionally, developing a community-based approach to research oversight can foster greater accountability and transparency. Engaging participants and community representatives in the oversight process solidifies trust and ensures that the perspectives of those impacted by research are taken into account. By empowering communities and maintaining a clear dialogue about the role of IRBs and patient safety, researchers can build a robust framework that withstands the challenges posed by funding disruptions.
Advocating for Ethical Standards in Clinical Research
In the ever-evolving landscape of medical research, advocating for unwavering ethical standards is paramount. This advocacy is especially crucial during times of funding uncertainty when ethical considerations may be overshadowed by fiscal pressures. Researchers, institutions, and regulatory bodies must work collaboratively to reaffirm their commitment to ethical research practices. By prioritizing patient safety and adhering to established ethical guidelines, the medical research community can safeguard participants’ rights and reinforce public trust.
Furthermore, integrating discussions about medical ethics into training programs for researchers is essential. Ensuring that those involved in clinical studies understand the historical context and significance of ethical standards will serve to uphold the integrity of the research enterprise. By fostering a culture of advocacy for ethical practices, we can better prepare the next generation of researchers to prioritize patient welfare and adhere to the highest standards of medical ethics.
The Future of Patient Safety and Research Funding
The trajectory of patient safety in medical research is closely tied to the stability of research funding. As financial resources continue to face cuts, the implications for patient protection and oversight protocols grow increasingly concerning. Funding must not only be restored but also expanded to enhance the systems that protect research participants. To ensure that the integrity of clinical trials is upheld, a commitment to adequate funding for IRBs and research ethics bodies is essential. Recognizing the link between funding and ethical oversight will pave the way for a more secure future in medical research.
Moreover, engaging policymakers and stakeholders to discuss the critical importance of research funding can lead to initiatives that prioritize patient safety at every turn. Advocacy efforts must emphasize how funding supports vital oversight mechanisms that protect participants and promotes the integrity of research practices. By creating a collective voice that prioritizes patient safety and the ethical conduct of research, the medical research community can navigate the challenges posed by funding disruptions and ensure a future where patient welfare is always the highest priority.
Frequently Asked Questions
What is the IRB process and its significance in medical research oversight?
The IRB process, or Institutional Review Board process, is a critical component of medical research oversight. It involves a thorough review of research proposals to ensure that they comply with ethical standards and regulations designed to protect the rights and welfare of research participants. Through this process, the IRB assesses potential risks, ensures informed consent, and evaluates the overall study design to safeguard patient safety in research.
How does NIH funding impact medical research oversight?
NIH funding plays a significant role in enhancing medical research oversight by requiring that research involving human subjects is reviewed by an IRB. This funding ensures that adequate resources are allocated to uphold patient safety in research, supporting compliance with ethical standards and regulations. Additionally, recent NIH policies have mandated the use of single IRBs for multisite studies, streamlining the oversight process while maintaining high ethical standards.
What role do IRBs play in ensuring patient safety in medical research?
IRBs are essential in safeguarding patient safety in medical research through their independent review of study protocols. They evaluate the ethical implications of research, including risk assessment, informed consent processes, and the monitoring of adverse events. This oversight helps protect participants from potential harm and ensures that research adheres to established medical ethics.
Why is medical ethics important in the context of medical research oversight?
Medical ethics are crucial in medical research oversight as they establish the moral principles guiding research involving human participants. These principles include respect for persons, beneficence, and justice, which ensure that participants are treated ethically and that their rights and welfare are prioritized. Adhering to medical ethics helps maintain public trust and confidence in the research process.
How can funding cuts affect clinical research integrity and patient safety?
Funding cuts can severely compromise clinical research integrity by hindering the ability of IRBs to function effectively, leading to lapses in oversight that may jeopardize patient safety in research. When studies are halted or delayed due to financial constraints, the risk of harm to participants increases, and the collaborative efforts necessary for ethical research practices can be undermined, eroding public trust in the research enterprise.
What impact do historical events have on the current standards of medical research oversight?
Historical events, such as unethical medical experiments and significant breaches of patient rights, have profoundly shaped current standards of medical research oversight. These events underscored the necessity for robust ethical oversight structures like IRBs to protect research participants. The lessons learned from past atrocities inform modern regulations and practices that prioritize patient safety and informed consent in all research activities.
| Key Point | Details |
|---|---|
| Funding Freeze | The Trump administration halted over $2 billion in federal research grants to Harvard, impacting multiple areas of medical research. |
| SMART IRB System | SMART IRB is a national system for overseeing multi-site medical research, disrupted by funding cuts. |
| IRB Role | Institutional Review Boards (IRBs) are crucial for ensuring patient safety, ethical compliance, and informed consent in medical research. |
| Historical Context | Historical unethical medical practices highlight the need for IRBs and strict oversight to protect research participants. |
| Impact of Funding Cuts | Cuts lead to halted studies, decreased patient safety oversight, and diminished public trust in medical research. |
Summary
Medical research oversight is vital for safeguarding participants involved in studies. The recent halt in funding has jeopardized these oversight efforts, exposing gaps that could adversely affect patient safety and ethical standards. Ensuring robust medical research oversight remains a priority as it plays a crucial role in maintaining public trust and the integrity of scientific inquiry.